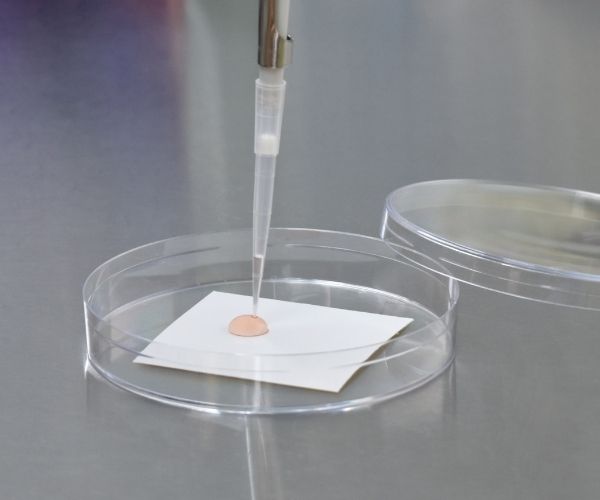
ISO 21702 is used to determine the antiviral activity of treated non-porous surfaces such as plastics. A 5 x 5 cm section of product is inoculated with the relevant virus, Influenza A and Feline calicivirus are referenced in the standard but the method can be adapted for other viruses. A 4 x 4 cm coverslip is placed on top to ensure contact with the active over the whole area. The sample and virus is then incubated at room temperature in a humidified chamber for 24 hours. The material and coverslip are then washed with a liquid neutraliser or similar to recover the remaining virus. The amount of virus surviving on the test article is quantified and the antiviral activity calculated.
- Required viruses: Influenza A (enveloped) and Feline calicivirus (non-enveloped). Feline calicivirus is used as a surrogate for Norovirus. Other viruses available on request.
- Sample requirements: 5 x 5 cm control and antiviral specimens (sterile)
- Contact time: 24 hours, other contact times can be added
- End point: TCID50
- Efficacy: Not set by standard, to be agreed on by all interested parties