Wound Care
Specialised testing for new and existing wound care products
Methods to Show Antimicrobial and Wound Healing Efficacy
Virologica has a variety of wound care methods that can be used for development and regulatory approval. If we don’t have the right method at the moment, we will work with you to develop one!
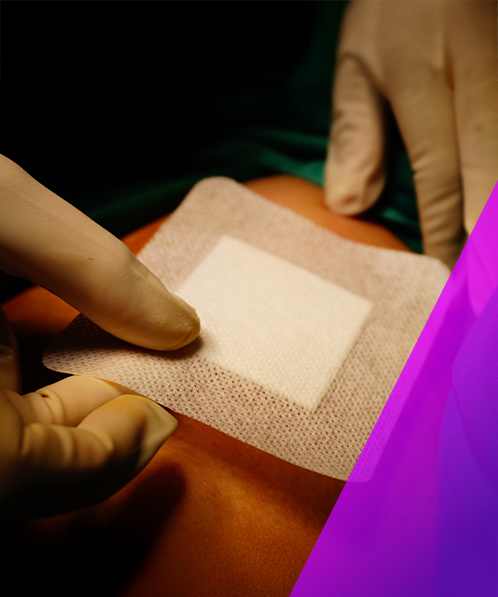
Testing Wound Care Products
Types of wounds
Wound care products are developed to support the process of healing wounds. Chronic wounds are wounds that fail to progress through the normal stages of healing and may not heal at all. There are risk factors that can contribute to this, for example diabetes and venous ulcers. For these patients, receiving proper and timely care for their wounds is critical. Another contributing factor to non-healing wounds can be infection by bacteria. Although most wounds are colonised by commensal bacteria, a higher bacterial load can lead to inflammation and trigger an immune response.
Testing of wound care products
Antimicrobial wound care products need to show efficacy against a range of bacteria and fungi. Antimicrobials can be administered as a dressing, gel or wash and a range of actives are seen on the market. There are some standard methods for testing efficacy as well as bespoke methods using ex vivo porcine skin which can closely mimic a wound without the need for a clinical study. There are also a range of proteins and other factors which may be elevated in non-healing wounds, for example matrix metalloproteinases (MMPs). These can be studied using commercially available ELISA kits to show sequestration that can aid wound healing.
Why Virologica
Providing quality, expert testing services
Knowledge
Our scientists understand the needs of wound care products and have the scientific knowledge to support you through your project.

Quality
We understand how important each test is to you and our team is committed to delivering high-quality data.
Flexible
We can work with you to adapt methods if your product does not exactly fit the standard.

Communication
We provide realistic timelines from the outset and keep you in the loop if anything changes.


Selection of Relevant Standards
AATCC Test Method 100 (modified)
Assessment of antibacterial finishes on textile materials (modified method requested by FDA)
ASTM E3383
Standard test method for determining the microbial barrier properties of wound dressing – in vitro wound model
ASTM E2799
Standard test method for testing disinfectant efficacy against Pseudomonas aeruginosa biofilm using the MBEC assay
What Our Customers Say…
“Customers needed to know that harmful viruses could be completely removed from our medical device prior to reuse. Virologica’s rigorous experimental protocol quickly delivered the clear and reliable evidence we needed.”
Technical Manager, Healthcare Business
Start The Conversation
Contact us today to explore how we can support you with your next project.